Abstract
Background:Venous thromboembolism (VTE) is a highly prevalent complication of cancer and its treatment and is commonly treated with anticoagulation1. However, there are limited data regarding the use of therapeutic anticoagulation in patients with brain metastases, due to concerns for intracranial hemorrhage (ICH).
Methods:We retrospectively identified cancer patients who were diagnosed with brain metastasis between 12/2005 and 7/2017, and subsequently underwent whole brain radiation at the Cleveland Clinic. Patients with primary brain tumors, leptomeningeal disease alone, and absence of follow-up brain imaging were excluded. Clinically significant ICH was defined as ICH resulting in focal neurologic deficit or required neurosurgery, as previously described2. Cumulative incidence of ICH from initial diagnosis of brain metastasis was calculated with death as competing risk. Difference between cumulative incidence estimates was tested using Gray's test. Overall survival (OS) calculated from initial diagnosis of brain metastasis, estimated by the Kaplan-Meier method, and compared by the log-rank test. Median follow-up time was calculated using reverse Kaplan-Meier method. Predictors identified as statistically significant on univariate logistic regression analysis (p<0.05) were selected for multivariate analysis.
Results:We screened568 patients and identified 407 who meet inclusion criteria. Seventy-eight (19%) patients received therapeutic anticoagulation (AC) [enoxaparin: n = 46 (59%), warfarin: n = 27 (35%), apixaban/rivaroxaban: n = 3 (4%), unfractionated heparin: n = 2 (2%)] due to VTE after diagnosis of brain metastasis, whereas 329 (81%) patients did not receive any therapeutic dose of AC. There were more female patients in the AC cohort, but other baseline characteristics were similar in both cohorts (Table 1).
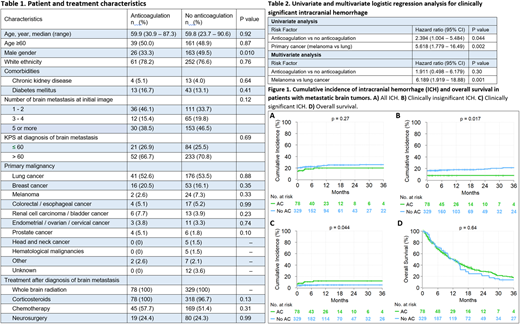
In the AC cohort, 11 of 78 (14%) patients had ICH, of which 6 (55%) were clinically insignificant and 5 (45%) were clinically significant at initial diagnosis of brain metastasis. Five of 11 patients were already on AC before diagnosis of brain metastasis. In the no-AC cohort, 65 of 329 (20%) patients had ICH, of which 53 (82%) clinically insignificant and 12 (18%) clinically significant at initial diagnosis of brain metastasis. Median follow-up of patients was 28 months. The 3-year cumulative incidence of clinically insignificant ICH in AC and no-AC groups was 7.7 (95% CI: 1.8 - 13.6) and 21.3 (95% CI: 16.5 - 26.1), respectively (p = 0.017). The 3-year cumulative incidence of clinically significant ICH in AC and no-AC groups was 12.0 (95% CI: 4.6 - 19.4) and 4.9 (95% CI: 2.6 - 7.4), respectively (p = 0.044). The 3-year cumulative incidence of all ICH in AC and no-AC groups was 19.7 (95% CI: 10.7 - 28.7) and 25.8 (95% CI: 20.9 - 30.7), respectively (p = 0.27). The 3-year OS in AC and no-AC groups was 13.6 (95% CI: 6.9 - 26.4) and 17.6 (95% CI: 12.9 - 23.9), respectively (p = 0.64) (Figure 1).
In univariable analysis, AC vs no-AC [OR: 2.39, 95% CI: 1.00 - 5.48, p = 0.044] and primary cancer (melanoma vs lung) [OR: 5.62, 95% CI: 1.78 - 16.49, p = 0.002] were predictors of clinically significant ICH after diagnosis of brain metastasis (Table 2). In multivariable analysis, only primary cancer (melanoma vs lung) [OR: 6.19, 95% CI: 1.92 - 18.88, p = 0.001]remained a statistically significant predictor of clinically significant ICH after diagnosis of brain metastasis.
Conclusion:
In this relatively large cohort of patients with brain metastasis, use of therapeutic anticoagulation did not influence the incidence of developing ICH but was associated with a greater risk of clinically significant ICH. This increase was, however, not linked to differences in overall survival. Nearly all patients in our AC cohort were treated with enoxaparin; ongoing studies will examine whether anticoagulation with DOACs is associated with similar outcomes.
Khorana:Bayer: Consultancy; Sanofi: Consultancy; Janssen: Consultancy; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal